1. Carbon Capturing: Methods and Techniques
Innovating with carbon capture: the key to unlocking new revenue streams


Which gases cause climate change?
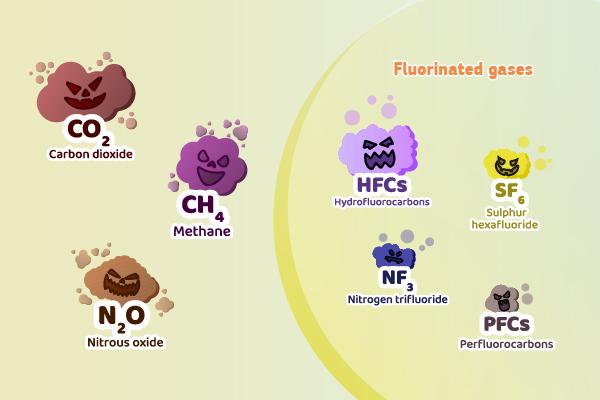
The most significant Greenhouse Gas (GHG) is carbon dioxide (CO2), which is emitted from a variety of sources, including energy production, transportation, industrial processes, and land use changes. The effect of carbon monoxide (CO) on the Earth's atmosphere was first confirmed in the late 19th century, as scientists began to understand the role of GHGs in the Earth's climate system.
Other GHGs include methane (CH4), which is emitted from livestock, waste, and the extraction and processing of fossil fuels; nitrous oxide (N2O), which is emitted from agriculture and the use of nitrogen-based fertilizers; and fluorinated gases, such as hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF6), which are emitted from a variety of industrial processes and refrigerants.
According to data from the United Nations Framework Convention on Climate Change (UNFCCC), global CO2 emissions have increased significantly over the past several decades, with the largest increase occurring since the 1980s. In 2020, global CO2 emissions reached a record high of 52.9 gigatons, an increase of more than 50% since 1990.
As I learned about the sources of greenhouse gas (GHG) emissions, I was shocked to discover that carbon dioxide (CO2) alone accounted for nearly 79% of global emissions in 2020. Methane (CH4) and nitrous oxide (N2O) were also significant contributors, at 14.1% and 6.0%, respectively. Even though they made up a smaller portion of overall emissions, fluorinated gases still contributed 1%.
When I looked at the top GHG emitters, I was unsurprised to see that China led the way, responsible for 28% of global emissions. The United States came in second with 14.6%, followed by India at 7.3%. The Russian Federation and Japan rounded out the top five, with 4.8% and 3.9% of total emissions, respectively.

I was interested to see that Canada, my home country, was the ninth largest GHG emitter in 2020, with 2.0% of global emissions. South America was the seventh largest contributor, at 3.2%, and Africa was the eighth largest, at 2.7%. It's clear that GHG emissions are a global problem, and it will take efforts from countries around the world to address it.
As I delved deeper into the topic of GHGs, I learned that different gases have different atmospheric lifetimes - in other words, the amount of time they stay in the atmosphere. Some, like carbon dioxide (CO2), can stick around for hundreds of years, while others, like methane (CH4), only last for about 12 years. However, I was surprised to learn that even though methane has a shorter lifetime, it is actually a more potent GHG, meaning it has a stronger warming effect on the Earth's surface.
I also learned about fluorinated gases, like hydrofluorocarbons (HFCs), perfluorocarbons (PFCs), and sulfur hexafluoride (SF6). These gases are often used in industrial processes and as refrigerants, and they can have global warming potentials that are hundreds or even thousands of times higher than carbon dioxide. And, unfortunately, they have long atmospheric lifetimes, ranging from 50 to thousands of years.
It's important to consider the atmospheric lifetime of GHGs when we think about ways to address climate change. Reducing emissions of long-lived gases like carbon dioxide can have a delayed but lasting impact on the Earth's climate, while reducing emissions of short-lived GHGs like methane can have a more immediate effect.
What the heck is Carbon Capture and Storage (CCS)?
As the world began to grapple with the issue of climate change in the early 20th century, scientists and policymakers started looking for ways to reduce greenhouse gas (GHG) emissions from large industrial sources. One solution that emerged was carbon capture and storage (CCS). The idea was to capture CO2 emissions from these sources and store them underground in a safe and permanent manner.
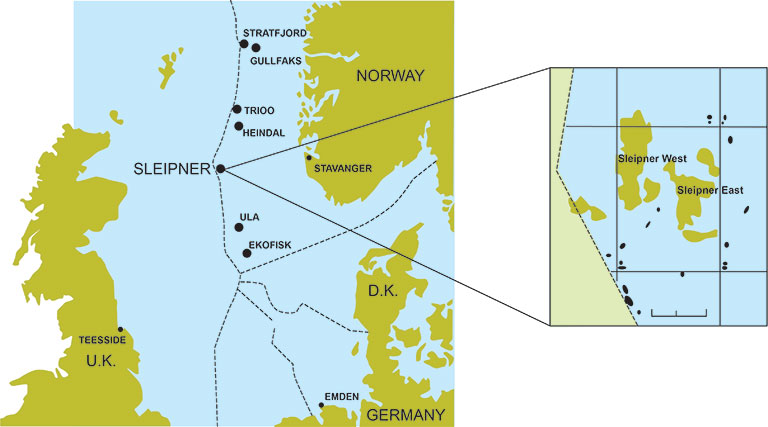
One of the first large-scale CCS projects was the Sleipner project in Norway. This project, which began operations in 1996, captured CO2 from a natural gas processing plant and stored it in a saline aquifer beneath the earth's surface.

How about Carbon Capture and Utilization (CCU)?
Another approach to reducing GHG emissions is carbon capture and utilization (CCU). CCU involves capturing CO2 from industrial sources and using it to produce valuable products, such as chemicals and fuels. CCU has been researched and developed for several decades, with the first projects starting in the 1980s.
One of the earliest CCU projects was the CO2 Recovery Plant at the Statoil Kårstø facility in Norway. This project, which began operating in the 1980s, captured CO2 from a natural gas processing plant and used it to produce methanol, a chemical that has a wide range of uses in industry and consumer products.

CCS and CCU combine into CCUS
In recent years, the term "carbon capture, utilization, and sequestration" (CCUS) has been used to describe the combination of carbon capture and storage (CCS) and carbon capture and utilization (CCU). These technologies are often discussed and implemented together as part of efforts to reduce greenhouse gas (GHG) emissions.
The reason for this is that CCS is an expensive process, and the product it produces is often too cheap to be worthwhile on its own. However, when carbon capture is combined with a utilization process that uses cheap CO2 to produce high-value chemicals, it becomes more economically viable. For example, the captured CO2 can be used to create new products, such as fuels and chemicals, which can be sold on the market and generate revenue.
CCS and CCUS are not just about protecting the environment; they also have the potential to create economic opportunities and drive innovation in a variety of industries. For example, CCS can be used to enhance oil and natural gas production, leading to increased profits for energy companies. However, injecting CO2 into geological formations for enhanced oil recovery or natural gas separation has been criticized for increasing emissions when the gas or oil is burned.
Currently, only a tiny fraction of global CO2 emissions are captured by carbon capture and storage (CCS). Most CCS projects focus on fossil gas processing, and the captured CO2 is either stored in deep geological formations or as mineral carbonates. Research is also being conducted on pyrogenic carbon capture and storage (PyCCS).
Geological formations are considered the most promising storage sites for CO2, and according to the US National Energy Technology Laboratory (NETL), North America alone has enough storage capacity for over 900 years of CO2 at current production rates. However, predicting the long-term security of underground or submarine storage is difficult and uncertain, and there is a risk of leakage into the atmosphere.
As we work towards a low-carbon future, it is essential that we continue to invest in CCS and carbon capture and utilization (CCSU) and find new and innovative ways to use these technologies. By doing so, we can not only help to protect the planet and reduce greenhouse gas emissions, but we can also create economic opportunities and drive innovation in a variety of industries.
CCUS Value Chain
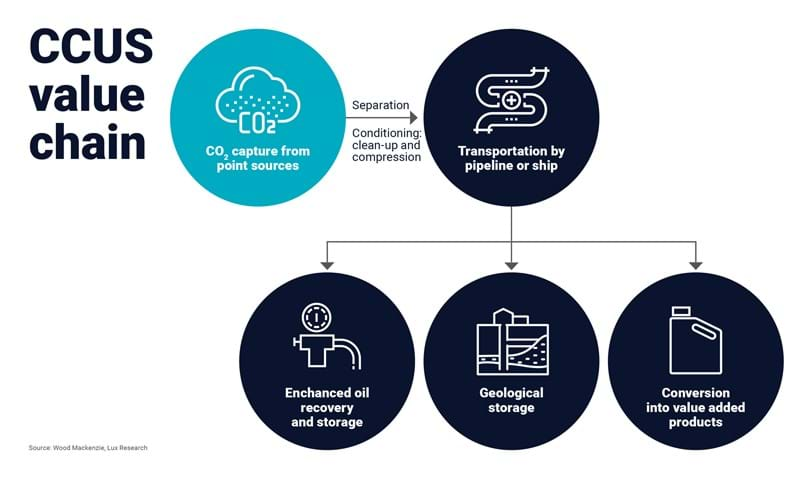
In this first article in our series on the benefits of carbon capture, utilization, and sequestration (CCUS) for the chemical industry, we will focus on the methods used for capturing carbon dioxide (CO2) emissions before they enter the atmosphere or from the atmosphere. This is a crucial step before we discuss the storage and utilization of these emissions and the products that can be made from these feedstocks. We hope you find this article informative and helpful in your understanding of CCUS and its potential for the chemical industry.
Have you ever stopped to think about how we actually go about capturing all of that CO2 that's released into the atmosphere from power plants and industrial facilities? It's not as simple as just sucking it all up with a giant vacuum cleaner. In fact, there are several different methods for capturing CO2, each with its own set of advantages and disadvantages.
According to the Global CCS Institute, as of 2021, there were 22 large-scale CCS facilities operating around the world, with an additional 11 facilities under construction or in advanced development.
Carbon Capture Methods and Separation Techniques
There are a few different ways to classify (or group them) carbon capture methods. One way is based on where the capture happens - before or after the fuel is burned, or during a chemical reaction that creates a stream of CO2 that can be easily captured. Another way is based on the type of technology used - some methods use chemicals, solid materials, or membranes to separate CO2 from other gases, while others rely on chemical reactions or water to react with CO2. Carbon capture can also be categorized based on the source of the CO2 - it can be captured from large industrial sources, specific point sources, or the exhaust gases of burning fossil fuels. Finally, carbon capture can be classified based on what happens to the captured CO2 - it can either be stored underground or used to create valuable chemicals or products. Energy consumption should also be considered when evaluating the effectiveness of different carbon capture methods.
The term "method" and "technique" can be used somewhat interchangeably to refer to a specific way of doing something. However, they can also have slightly different connotations.
A method is generally a systematic or standardized approach to achieving a particular goal. It is a set of steps or procedures that are followed in a specific order to achieve a desired result.
A technique, on the other hand, is a specific skill or method of doing something, often involving the use of a particular tool or process. Techniques are typically more specific and focused than methods, and may be used as part of a larger method or approach.
In the context of carbon capture and storage, "methods" might refer to broad approaches or technologies that are used to capture CO2 from the air or from industrial sources, such as pre-combustion capture or chemical absorption. "Techniques" might refer to specific skills or processes that are used as part of these methods, such as membrane separation or adsorption.
So, whether something is a method or a technique may depend on the level of specificity and the context in which it is being used.
Let’s look at them all one by one.
1. Pre-combustion capture:

This method involves separating hydrogen and CO2 in a fuel before it is burned, using a process called gasification. By capturing the CO2 before the fuel is combusted, pre-combustion capture has the potential to significantly reduce GHG emissions.
While researching this technology, I discovered that it has a number of pros and cons. One of the biggest advantages of pre-combustion capture is that it can be used with a variety of fossil fuels, including coal, natural gas, and biogas. This means that it has the potential to be widely applicable and useful in a variety of situations. Pre-combustion capture can also significantly reduce CO2 emissions, which is critical for addressing climate change. Additionally, the hydrogen produced through pre-combustion capture can be used as a clean-burning fuel, which has the potential to reduce air pollution and improve public health.
However, pre-combustion capture also has some challenges. It requires a high-pressure, high-temperature environment, which can be expensive to maintain. This means that the overall cost of using pre-combustion capture may be higher than some other CCS technologies. Additionally, pre-combustion capture can be technically challenging to implement. It requires specialized equipment and processes, and it can be difficult to ensure that it is working efficiently and effectively.
2. Oxy-fuel combustion capture:

This method involves capturing CO2 emissions by burning fuel in a mixture of oxygen and recycled flue gas, which produces a concentrated stream of CO2 that can be easily captured.
While researching this technology, I discovered that it has a number of pros and cons. One of the biggest advantages of oxy-fuel combustion capture is its high capture efficiency; it can capture up to 90% of CO2 emissions. This is much higher than some other CCS technologies. Oxy-fuel combustion capture also produces a concentrated stream of CO2 that is easy to capture and store, which can make it more practical and cost-effective to implement. Additionally, oxy-fuel combustion capture can be retrofitted to existing power plants, which means that we don't have to build new facilities in order to use this technology.
However, oxy-fuel combustion capture also has some challenges. It requires the use of pure oxygen, which can be expensive to produce. This means that the overall cost of using oxy-fuel combustion capture may be higher than some other CCS technologies. Additionally, oxy-fuel combustion capture can be technically challenging to implement, especially in large-scale power plants. This means that it may require specialized equipment and processes, and it may take longer to get up and running than some other CCS technologies.
3. Post-combustion capture:

As I researched carbon capture and storage (CCS) technologies, I learned about post-combustion capture. This method involves capturing CO2 emissions from the flue gas produced by burning fossil fuels. It has both advantages and disadvantages.
One of the things that I found most appealing about post-combustion capture is that it can be retrofitted to existing power plants and other industrial facilities. This means that we don't have to build new facilities from scratch in order to use this technology. Additionally, post-combustion capture can capture a wide range of gases, including CO2, methane, and other greenhouse gases. It can be used with a variety of fuels, including coal, natural gas, and biomass.
However, post-combustion capture also has its challenges. It can be energy-intensive, which can increase the overall cost of the process. This means that it can be more expensive to use post-combustion capture than some other CCS technologies. Additionally, post-combustion capture can be technically challenging to implement. It requires specialized equipment and processes, and it can be difficult to ensure that it is working efficiently and effectively.
4. Carbon capture from the air (DAC):

As I delved deeper into the world of carbon capture and storage (CCS) technologies, I came across a fascinating method called carbon capture from the air (DAC). This method involves using a machine to directly capture CO2 from the air. Almost a giant vacuum cleaner:)
I was immediately struck by the potential benefits of DAC. One of the main advantages is that it can capture CO2 from the atmosphere, rather than just from industrial facilities. This means that it has the potential to be a much more widespread and effective tool.
But that's not all - as I continued to research DAC, I learned that it has the potential to be a mobile and on-demand technology. This means that it can be deployed wherever it is needed, and it can be turned on and off as needed to meet changing demands. This makes DAC incredibly flexible and adaptable, and it could potentially be used in a wide variety of settings and create a lot of independent service providers. I am biased and love entrepreneurship.
However, DAC is not without its challenges. One of the main challenges is that it can be expensive to implement on a large scale. This means that it may be difficult to convince governments and businesses to invest in DAC projects. Additionally, DAC requires a lot of energy to operate, which can increase the overall carbon footprint of the process. This means that it is important to consider the overall environmental impact of DAC and ensure that it is used in a way that is sustainable and responsible.
Despite these challenges, I believe that DAC is a promising technology If we can overcome the technical and financial challenges. Its ability to be mobile and on-demand makes it an even more appealing option, and I think it is worth investing in this technology and finding ways to make it more cost-effective and energy-efficient.
5. Electrochemical capture:
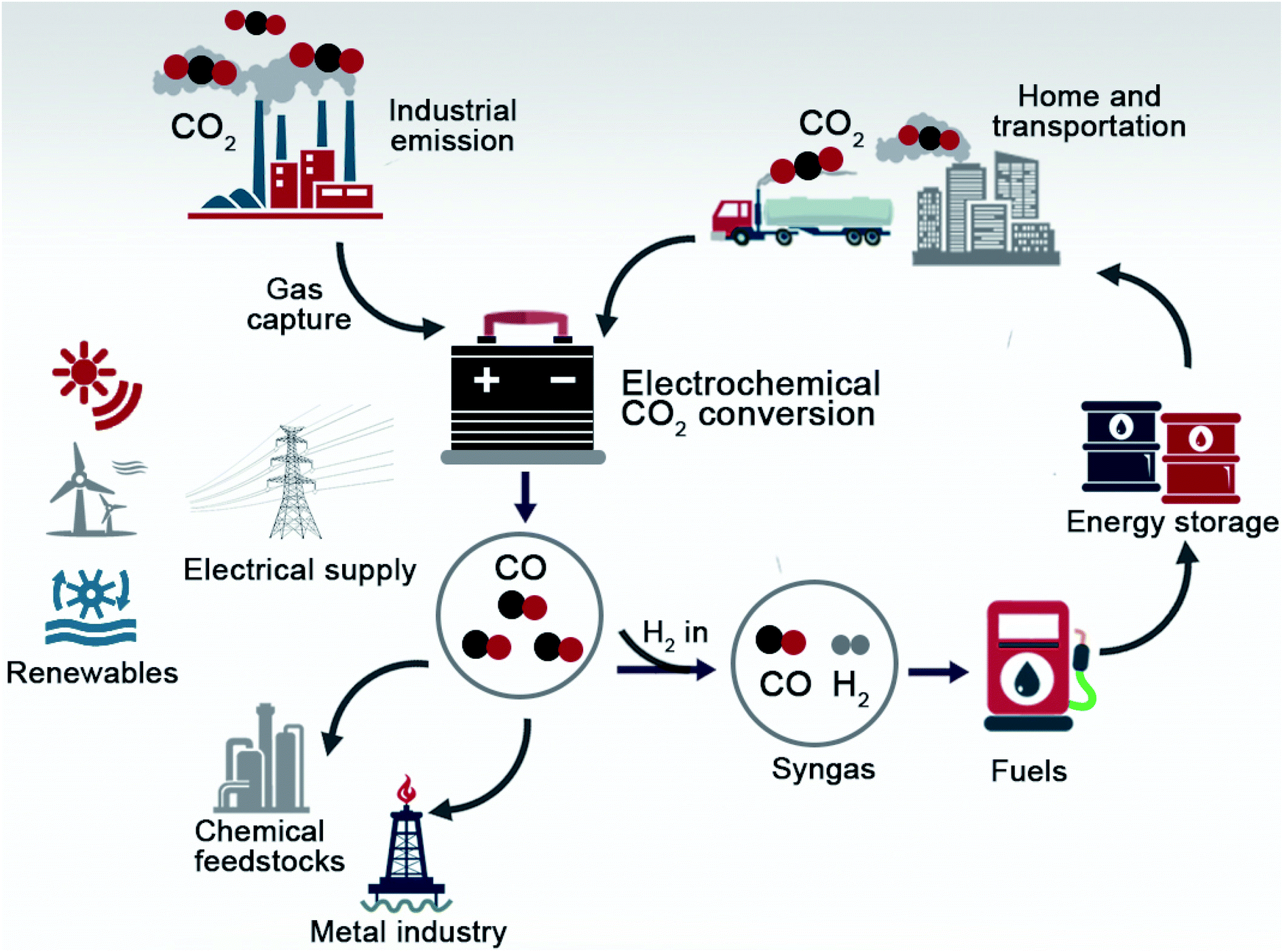
As I continued to explore the world of carbon capture and storage (CCS) technologies, I came across a promising method called electrochemical capture. This method involves using an electrochemical process to capture CO2 from the flue gas produced by burning fossil fuels.
I was immediately intrigued by the potential benefits of electrochemical capture. One of the main advantages is that it can be retrofitted to existing power plants and other industrial facilities. This means that it has the potential to be a relatively low-cost and low-risk way of reducing GHG emissions. Additionally, electrochemical capture can capture a wide range of gases, including CO2, methane, and other greenhouse gases. This makes it a potentially powerful tool for addressing a variety of environmental issues.
As I learned more about electrochemical capture, I also discovered that it has the potential to be more energy-efficient than other CCS methods. This is an important consideration, as the energy required to capture and store CO2 can be a significant portion of the overall cost of CCS.
However, as I continued to research electrochemical capture, I also learned about some of the challenges it faces. One of the main challenges is that it is still in the early stages of development and has not yet been demonstrated at a commercial scale. This means that it will require significant investment and research in order to bring it to market. Additionally, electrochemical capture requires the development of new materials and technologies, which can be technically challenging and costly.
6. Bioenergy with carbon capture and storage (BECCS):
Have you heard of bioenergy with carbon capture and storage (BECCS)? It's a technology that captures CO2 from the flue gas produced by burning biomass, such as wood or agricultural waste, and stores it underground. I think it has some really interesting potential as a tool for combating climate change.
One of the things that I like about BECCS is that it can be used with a variety of biomass feedstocks. That means it can be tailored to local resources and conditions, which is really important when it comes to finding solutions that work for different communities around the world. Plus, BECCS has the potential to be carbon-negative, which means it could remove more CO2 from the atmosphere than it produces.
Of course, there are also some challenges to BECCS. It's technically challenging to implement, and it requires a reliable supply of biomass feedstocks. And it can be expensive to roll out on a large scale, which might make it harder to convince governments and businesses to invest in it.
7. Direct air capture with chemical looping (DACCL):
It's a pretty cool technology that's being developed to help combat climate change. DACCL uses a chemical reaction to capture CO2 from the air to produce a fuel or chemical.
Now, you might be thinking "wait a minute, isn't direct air capture just capturing CO2 from the air?" And you'd be right! But here's the thing: DACCL has some key differences that make it stand out.
One of the main advantages of DACCL is that it has the potential to be more energy-efficient and to produce a valuable product that can be sold on the market, which could help offset the cost of implementing the technology. This is a nice added bonus because let's face it, everyone loves a good product that can generate some extra revenue.
Now, DACCL is still in the early stages of development and hasn't been demonstrated at a commercial scale yet. This means that it will require some more research and development before it's ready for the big leagues. It also requires the development of new materials and technologies, which can be technically challenging and costly. Everything is costly at the beginning, but without those initial investments, we wouldn’t see a lot of things we enjoy at a very low price right now.
8. Membrane separation:
It's a pretty neat technology that's being developed to help reduce GHG emissions. The basic idea is to use a membrane to separate CO2 from the other gases in the flue gas.
So, what are the pros of using this method? For starters, it can be used to capture a wide range of gases, including CO2, methane, and other greenhouse gases. That's a big deal because it means that this technology has the potential to be used in a variety of different industries. Additionally, membrane separation can be retrofitted to existing power plants and industrial facilities, which makes it a flexible and adaptable technology.
But there are also some cons to consider. One of the main challenges of membrane separation is that the membranes can be expensive to manufacture and maintain. This can be a barrier to widespread adoption of the technology, especially if it's not cost-effective.
9. Solvent-based capture:
And if you thought membrane separation was cool, wait until you hear about solvent-based capture! This method involves using a chemical solvent to absorb CO2 from flue gas. Once the CO2 is absorbed, it can be released and captured when the solvent is regenerated.
One of the main advantages of solvent-based capture is that it can capture CO2 from flue gas in a relatively simple and energy-efficient process. That's a big deal because it means that this technology has the potential to be an affordable and effective option. Additionally, solvent-based capture can be retrofitted to existing power plants and industrial facilities, which makes it a flexible and adaptable technology.
However, there are also some cons to consider. One of the main challenges of solvent-based capture is that the solvents used in the process can be expensive and may need to be replaced periodically. This can be a barrier to the widespread adoption of the technology, especially if it's not cost-effective.
10. Adsorption-based capture:
And if you're still with me, let's talk about adsorption-based capture! This method involves using a porous material to adsorb CO2 from flue gas. Once the CO2 is adsorbed, it can be released and captured when the material is regenerated.
Like solvent-based capture, adsorption-based capture has the potential to capture CO2 from flue gas in a relatively simple and energy-efficient process. It can also be retrofitted to existing power plants and industrial facilities, which makes it a flexible and adaptable technology.
And like solvent-based capture, one of the main limitations of adsorption-based capture is that the adsorbent materials used in the process can be expensive and may need to be replaced periodically. This can be a barrier to the widespread adoption of the technology, especially if it's not cost-effective.
11. Algae-based capture:
And let's not forget about algae-based capture! This method involves using algae to absorb CO2 from the air or flue gas. Once the CO2 is absorbed, the algae can be harvested and used to produce biofuels or other products.
One of the main advantages of algae-based capture is that it has the potential to capture CO2 from the air or flue gas in a relatively simple and energy-efficient process. It also has the potential to produce valuable products, which could create economic opportunities and drive innovation in a variety of industries.
However, there are also some challenges to consider. One of the main limitations of algae-based capture is that the technology is still in the early stages of development and has not yet been tested at a large scale. Additionally, the algae used in the process may need to be grown and harvested using energy-intensive methods, which could increase the overall carbon footprint of the process.
New methods to capture CO2 emissions
There are several new and experimental methods for capturing CO2 emissions that are being researched or developed by universities and companies around the world. Some of these methods include:
1. Photocatalytic capture:
This method involves using a photocatalyst, such as titanium dioxide, to convert CO2 into a solid form that can be easily captured and stored or to catalyze the conversion of CO2 into a chemical compound, such as formic acid or methanol. The CO2 is typically mixed with a solvent and then exposed to the photocatalyst, which causes the CO2 to react with the solvent and form a stable compound. The resulting compound can then be separated from the solvent and stored, or it can be used to produce other chemicals or fuels. One potential advantage of photocatalytic capture is that it can be powered by sunlight, which is a renewable and widely available energy source.
2. Ion transport membrane capture:
This method involves using a membrane to selectively transport ions, such as CO2 ions, from a gas mixture, allowing them to be captured and stored. It basically uses a membrane to separate CO2 from other gases in a flue gas stream. The membrane is made of a material that is selective for CO2, meaning that it is more permeable to CO2 than other gases. When the flue gas is passed through the membrane, the CO2 is transported through the membrane and collected on the other side, while the other gases are left behind. It has the potential to be an energy-efficient method for capturing CO2, as it does not require the use of solvents or other chemicals. It may also be suitable for use in a variety of industries, including power generation, cement production, and chemical processing. There are several challenges that must be addressed in order to make ion transport membrane capture a viable option for carbon capture and storage (CCS). These include developing durable, selective membranes that can withstand the high temperatures and pressures of a flue gas stream, and finding ways to minimize the energy required to operate the system. Researchers are working on these and other issues to improve the performance and feasibility of ion transport membrane capture.
3. Hollow fiber membrane capture:
This method involves using a membrane with hollow fibers to capture CO2 from flue gas or the air, while the remaining gas is released. This technology has several potential advantages, including its ability to operate at low pressure and its potential for high CO2 capture efficiency. However, it is still in the early stages of development. Some of the challenges that need to be overcome include optimizing the design of the membrane to maximize CO2 capture efficiency and minimizing the cost of the process. There are several researchers and institutions that are currently working on the development of hollow fiber membrane capture technology, including the University of Kentucky and the Massachusetts Institute of Technology (MIT).
4. Metal-organic framework (MOF) capture:
This method involves using a porous material known as a metal-organic framework to capture CO2 from flue gas or the air. The CO2 is then released and captured when the MOF is regenerated. MOFs are made up of metal ions connected by organic molecules, creating a porous and highly specific material with a large surface area for adsorption. They have been shown to have high selectivity for CO2 over other gases, making them an attractive option for carbon capture. However, there are still many challenges to be overcome in order to make MOF capture a viable technology, including improving the stability and durability of the MOFs, developing efficient methods for their regeneration, and reducing the cost of production. Some researchers are also exploring the use of other porous materials, such as zeolites and activated carbon, for carbon capture. These materials have the advantage of being well-studied and widely available, but they may not be as selective for CO2 as MOFs.
5. Electrified liquid capture:
This method involves using an electrified liquid to capture CO2 from flue gas or the air. The CO2 is attracted to the electrified liquid due to the Coulombic forces between the charged particles and can be separated from the gas stream by applying an electric field. This technology has the potential to be more energy-efficient and cost-effective than other CCS methods, as it does not require the use of solvents or other chemicals. However, there is limited information available on electrified liquid capture as a method for capturing CO2 emissions. It is a relatively new and experimental approach that is still in the early stages of development.
6. Plasma-based capture:
This method involves using plasma to convert CO2 into a solid form that can be easily captured and stored. The CO2 is attracted to the plasma by an electric field and then cooled, condensed, and collected. Some of the challenges that need to be addressed include the development of robust, long-lasting plasma sources and the management of the large amounts of energy required to create and maintain the plasma
7. Electrostatic capture:
This method involves using an electrostatic field to capture CO2 from flue gas or the air. Some of the potential advantages of electrostatic capture include its relatively simple and energy-efficient operation, and its ability to capture a wide range of gases, including CO2, methane, and other greenhouse gases. However, there are also some challenges to overcome, such as the development of suitable materials for the capture process and the cost of implementing the technology on a large scale. Some research groups and private companies are currently working on developing and testing electrostatic capture systems, and it is possible that this technology could be used in the future to help reduce CO2 emissions from power plants and other industrial facilities.
8. Solid-state capture:
This method involves using a solid material to capture CO2 from flue gas or the air. One of the main advantages of this approach is that the solid materials used in the process can be regenerated, allowing them to be used repeatedly. This means that the overall cost of the process could potentially be lower than other carbon capture technologies. One of the main challenges of solid-state carbon capture is finding materials that are able to effectively capture CO2 while also being stable and long-lasting. Researchers are still working on developing new materials that can be used in this process, and it is not yet clear which materials will be most effective. One potential application of solid-state carbon capture is in the production of hydrogen fuel. By capturing CO2 from the flue gas produced by burning fossil fuels, it may be possible to produce hydrogen fuel in a more sustainable and environmentally friendly way.
9. Photobiological capture:
This method involves using photosynthetic microorganisms, such as cyanobacteria, to capture CO 2 from the air or flue gas. The CO2 is then converted into a form that can be stored or used to produce a valuable product, such as biofuels or chemicals. Some of the challenges that need to be addressed include finding ways to optimize the growth and productivity of the microorganisms, as well as developing cost-effective methods for capturing and storing the CO2 that is absorbed. Despite these challenges, photobiological carbon capture has the potential to be a relatively simple and energy-efficient method for capturing CO2 emissions. It may also have the added benefit of producing valuable products that can offset the cost of the capture process.
10. Molecular capture:
This method refers to the use of small molecules or chemical compounds to capture CO2 from flue gas or the atmosphere. These molecules or polymers are designed to bind specifically to CO2, allowing them to be selectively removed from a gas stream. The captured CO2 can then be released by heating the material or applying a chemical trigger and can be stored or used to make other products. Some potential advantages of molecular capture include the ability to selectively capture CO2 without the need for high-pressure or high-temperature conditions, the potential to be integrated into existing industrial processes, and the possibility of using the captured CO2 as a feedstock for the production of chemicals or fuels. However, there are also challenges that need to be addressed in the development of molecular capture technologies, including the need to design stable and selective molecules that can be used over multiple cycles, the need to find suitable storage options for the captured CO2, and the need to develop efficient and cost-effective methods for regenerating the capture molecules.
11. Calcium looping capture:
This method involves using a chemical reaction between calcium oxide and CO2 to capture and store CO2. Calcium looping technology has a high potential for capturing CO2 in cement plants as the CaO-rich purge from the calciner can be used to replace a sizable fraction of the CaCO3 used as feedstock.
Conclusion
There isn't a single method that works for every carbon capture project. The right approach depends on various factors, such as the goals of the project, budget constraints, location, and available resources. Sometimes, multiple methods may be used in combination to achieve the desired results. However, it's important to consider the economic benefits of a project, as projects without financial benefits may struggle to secure funding or may not be sustainable in the long term.
So, next time you hear about carbon capture and storage (CCS), just remember that it's not as straightforward as it might seem.
Tackling climate change and finding ways to reduce carbon emissions is no small feat, and it takes a lot of brains and resources to come up with innovative solutions. That's why teams of scientists, engineers, and other experts from all sorts of fields are constantly working on new ways to capture carbon dioxide (CO2) emissions. From universities and research institutes to private companies, there are many organizations dedicated to finding the next big thing in carbon capture. And to make sure these projects have the resources they need to succeed; governments and other organizations often offer funding and support. So, if you've ever wanted to be a part of breakthrough, now's your chance!